Expo
view channel
view channel
view channel
view channel
view channel
view channel
view channel
MRIUltrasoundNuclear MedicineGeneral/Advanced ImagingImaging ITIndustry News
Events

- AI Can Flag Mammograms for Supplemental MRI
- 3D CT Imaging from Single X-Ray Projection Reduces Radiation Exposure
- AI Method Accurately Predicts Breast Cancer Risk by Analyzing Multiple Mammograms
- Printable Organic X-Ray Sensors Could Transform Treatment for Cancer Patients
- Highly Sensitive, Foldable Detector to Make X-Rays Safer
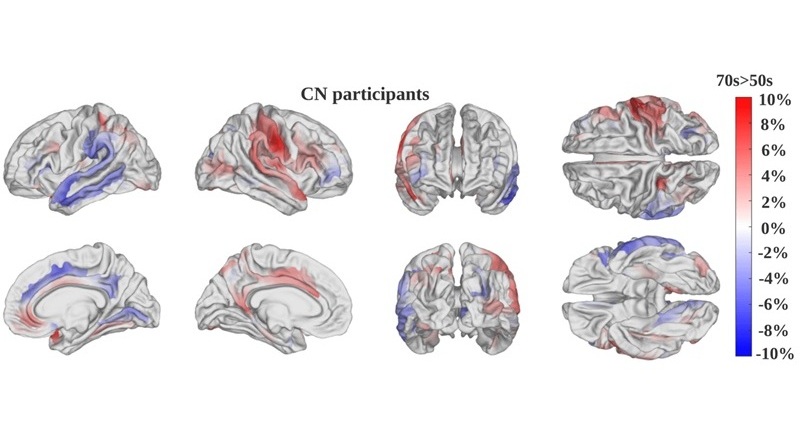
- First-Of-Its-Kind Tool Analyzes MRI Scans to Measure Brain Aging
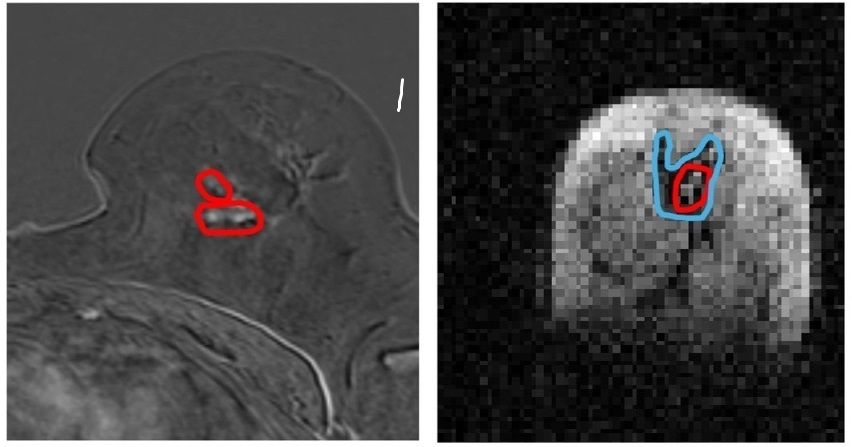
- AI-Enhanced MRI Images Make Cancerous Breast Tissue Glow
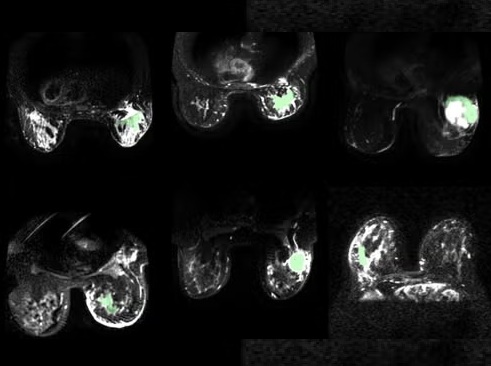
- AI Model Automatically Segments MRI Images
- New Research Supports Routine Brain MRI Screening in Asymptomatic Late-Stage Breast Cancer Patients
- Revolutionary Portable Device Performs Rapid MRI-Based Stroke Imaging at Patient's Bedside
- Next-Gen Tau Radiotracers Outperform FDA-Approved Imaging Agents in Detecting Alzheimer’s
- Breakthrough Method Detects Inflammation in Body Using PET Imaging
- Advanced Imaging Reveals Hidden Metastases in High-Risk Prostate Cancer Patients
- Combining Advanced Imaging Technologies Offers Breakthrough in Glioblastoma Treatment
- New Molecular Imaging Agent Accurately Identifies Crucial Cancer Biomarker
- AI Improves Detection of Congenital Heart Defects on Routine Prenatal Ultrasounds
- AI Diagnoses Lung Diseases from Ultrasound Videos with 96.57% Accuracy
- New Contrast Agent for Ultrasound Imaging Ensures Affordable and Safer Medical Diagnostics
- Ultrasound-Directed Microbubbles Boost Immune Response Against Tumors
- POC Ultrasound Enhances Early Pregnancy Care and Cuts Emergency Visits
- New CT Scan Technique to Improve Prognosis and Treatments for Head and Neck Cancers
- World’s First Mobile Whole-Body CT Scanner to Provide Diagnostics at POC
- Comprehensive CT Scans Could Identify Atherosclerosis Among Lung Cancer Patients
- AI Improves Detection of Colorectal Cancer on Routine Abdominopelvic CT Scans
- Super-Resolution Technology Enhances Clinical Bone Imaging to Predict Osteoporotic Fracture Risk
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Bracco Diagnostics and ColoWatch Partner to Expand Availability CRC Screening Tests Using Virtual Colonoscopy
- Mindray Partners with TeleRay to Streamline Ultrasound Delivery
- Philips and Medtronic Partner on Stroke Care
- Siemens and Medtronic Enter into Global Partnership for Advancing Spine Care Imaging Technologies
- RSNA 2024 Technical Exhibits to Showcase Latest Advances in Radiology

Expo
 view channel
view channel
view channel
view channel
view channel
view channel
view channel
MRIUltrasoundNuclear MedicineGeneral/Advanced ImagingImaging ITIndustry News
Events
Advertise with Us
view channel
view channel
view channel
view channel
view channel
view channel
view channel
MRIUltrasoundNuclear MedicineGeneral/Advanced ImagingImaging ITIndustry News
Events
Advertise with Us


- AI Can Flag Mammograms for Supplemental MRI
- 3D CT Imaging from Single X-Ray Projection Reduces Radiation Exposure
- AI Method Accurately Predicts Breast Cancer Risk by Analyzing Multiple Mammograms
- Printable Organic X-Ray Sensors Could Transform Treatment for Cancer Patients
- Highly Sensitive, Foldable Detector to Make X-Rays Safer
- First-Of-Its-Kind Tool Analyzes MRI Scans to Measure Brain Aging
- AI-Enhanced MRI Images Make Cancerous Breast Tissue Glow
- AI Model Automatically Segments MRI Images
- New Research Supports Routine Brain MRI Screening in Asymptomatic Late-Stage Breast Cancer Patients
- Revolutionary Portable Device Performs Rapid MRI-Based Stroke Imaging at Patient's Bedside
- Next-Gen Tau Radiotracers Outperform FDA-Approved Imaging Agents in Detecting Alzheimer’s
- Breakthrough Method Detects Inflammation in Body Using PET Imaging
- Advanced Imaging Reveals Hidden Metastases in High-Risk Prostate Cancer Patients
- Combining Advanced Imaging Technologies Offers Breakthrough in Glioblastoma Treatment
- New Molecular Imaging Agent Accurately Identifies Crucial Cancer Biomarker
- AI Improves Detection of Congenital Heart Defects on Routine Prenatal Ultrasounds
- AI Diagnoses Lung Diseases from Ultrasound Videos with 96.57% Accuracy
- New Contrast Agent for Ultrasound Imaging Ensures Affordable and Safer Medical Diagnostics
- Ultrasound-Directed Microbubbles Boost Immune Response Against Tumors
- POC Ultrasound Enhances Early Pregnancy Care and Cuts Emergency Visits
- New CT Scan Technique to Improve Prognosis and Treatments for Head and Neck Cancers
- World’s First Mobile Whole-Body CT Scanner to Provide Diagnostics at POC
- Comprehensive CT Scans Could Identify Atherosclerosis Among Lung Cancer Patients
- AI Improves Detection of Colorectal Cancer on Routine Abdominopelvic CT Scans
- Super-Resolution Technology Enhances Clinical Bone Imaging to Predict Osteoporotic Fracture Risk
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Bracco Diagnostics and ColoWatch Partner to Expand Availability CRC Screening Tests Using Virtual Colonoscopy
- Mindray Partners with TeleRay to Streamline Ultrasound Delivery
- Philips and Medtronic Partner on Stroke Care
- Siemens and Medtronic Enter into Global Partnership for Advancing Spine Care Imaging Technologies
- RSNA 2024 Technical Exhibits to Showcase Latest Advances in Radiology


















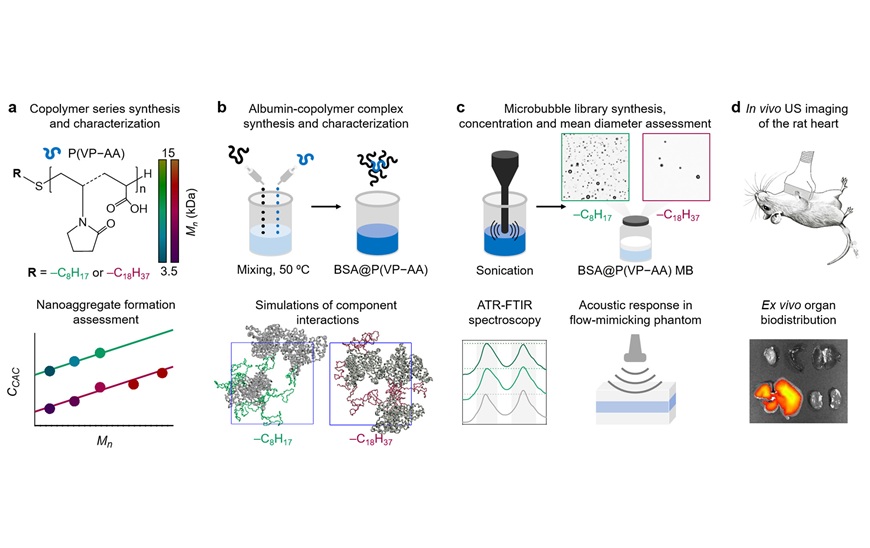
![Image: [18F]3F4AP in a human subject after mild incomplete spinal cord injury (Photo courtesy of The Journal of Nuclear Medicine, DOI:10.2967/jnumed.124.268242) Image: [18F]3F4AP in a human subject after mild incomplete spinal cord injury (Photo courtesy of The Journal of Nuclear Medicine, DOI:10.2967/jnumed.124.268242)](https://globetechcdn.com/medicalimaging/images/stories/articles/article_images/2025-02-24/Brugarolas_F8.large.jpg)
![Image: Autoradiography images showing binding of [18F]flortaucipir, [18F]MK6240, and [18F]PI2620 in prefrontal cortex, hippocampus, and cerebellum (A) and in whole-brain hemisphere (B) of control and AD brains (Photo courtesy of UFRGS) Image: Autoradiography images showing binding of [18F]flortaucipir, [18F]MK6240, and [18F]PI2620 in prefrontal cortex, hippocampus, and cerebellum (A) and in whole-brain hemisphere (B) of control and AD brains (Photo courtesy of UFRGS)](https://globetechcdn.com/medicalimaging/images/stories/articles/article_images/2025-02-12/F2.large.jpg)
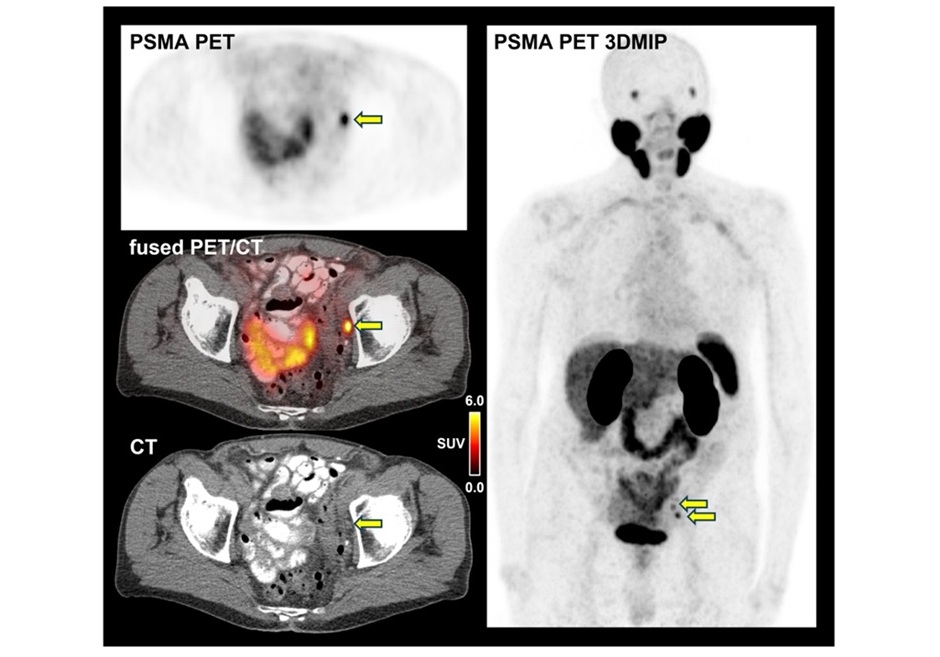
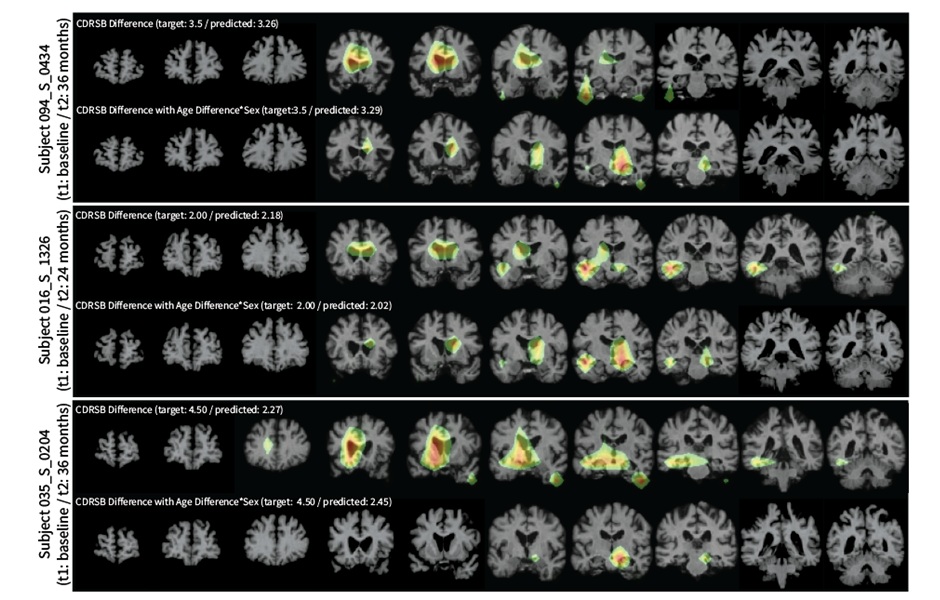





.jpg)
.jpeg)



